Introduction
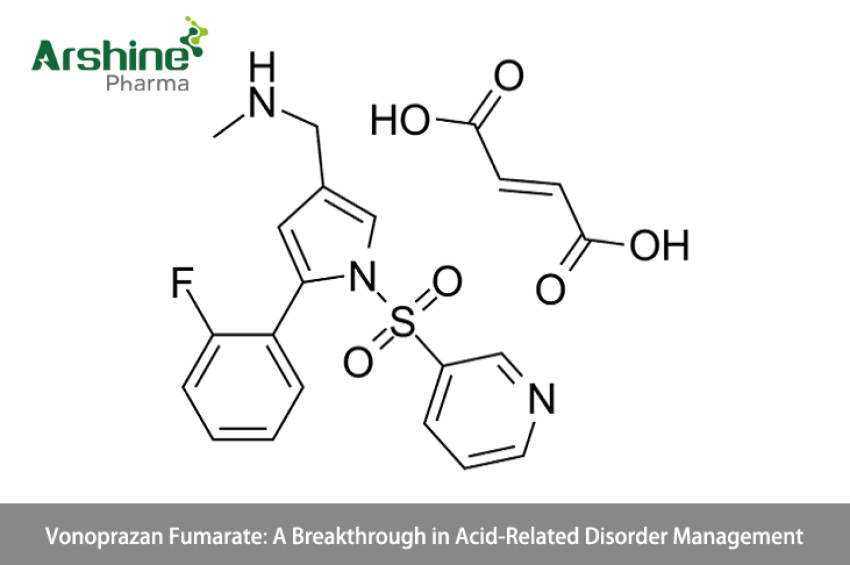
Vonoprazan fumarate is a novel proton pump inhibitor (PPI) that has garnered attention for its distinct mechanism of action and potential to revolutionize the treatment of acid-related disorders. With its ability to provide rapid and sustained acid suppression, Vonoprazan presents an exciting alternative to traditional PPIs, addressing the limitations of existing therapies. This article explores the mechanism of action, therapeutic indications, clinical efficacy, and potential impact of Vonoprazan fumarate in the field of gastroenterology.
Mechanism of Action
Unlike conventional PPIs that target the final step of gastric acid secretion by inhibiting the H+/K+-ATPase proton pump, Vonoprazan fumarate uniquely targets the potassium-competitive acid blocker (P-CAB) class. It selectively and reversibly inhibits the proton pump by binding to its potassium-binding site, ensuring continuous and profound acid suppression. This novel mechanism offers a faster onset of action and sustained acid control, setting Vonoprazan apart from its predecessors.
Therapeutic Indications
Vonoprazan fumarate's wide range of therapeutic applications extends to various acid-related disorders. It has shown efficacy in treating gastroesophageal reflux disease (GERD), peptic ulcers, Helicobacter pylori eradication, and Zollinger-Ellison syndrome. Its rapid and potent acid suppression makes it particularly valuable in managing refractory cases or those requiring more immediate relief. Additionally, Vonoprazan's potential in H. pylori eradication regimens could enhance treatment success rates and reduce antibiotic resistance concerns.
Clinical Efficacy
Clinical trials have highlighted Vonoprazan fumarate's superiority over conventional PPIs in terms of acid suppression and symptom relief. Studies comparing Vonoprazan with other PPIs in GERD management have demonstrated faster healing of erosive esophagitis and higher rates of symptom resolution. The drug's sustained acid suppression also proves beneficial in preventing ulcer recurrence, as observed in peptic ulcer trials. Furthermore, Vonoprazan's role in H. pylori eradication regimens has shown promising results, potentially shortening treatment duration and increasing eradication rates.
Potential Impact
The introduction of Vonoprazan fumarate addresses several challenges associated with traditional PPI therapy. Its rapid onset of action and sustained acid suppression could translate to quicker symptom relief and improved patient adherence. For individuals with difficult-to-treat or refractory acid-related disorders, Vonoprazan offers a valuable alternative that may enhance overall therapeutic outcomes. Its use in H. pylori eradication regimens also holds promise for reducing treatment failure rates and mitigating antibiotic resistance concerns.
Safety Profile
Vonoprazan fumarate's safety profile aligns with that of conventional PPIs, with mild and transient adverse effects such as headache, diarrhea, and abdominal pain reported in clinical trials. Long-term safety data are still being amassed, and ongoing monitoring will be crucial to comprehensively assess any potential risks associated with Vonoprazan use.
Conclusion
Vonoprazan fumarate's emergence as a potassium-competitive acid blocker marks a significant milestone in acid-related disorder management. Its unique mechanism of action, rapid onset of action, sustained acid suppression, and potential benefits in H. pylori eradication regimens position it as a promising addition to the gastroenterologist's toolkit. As further research and clinical experience accumulate, Vonoprazan's role and impact on patient care will continue to evolve, potentially transforming the landscape of acid-related disorder treatment.
https://www.arshinepharma.com/new/Vonoprazan-Fumarate



